
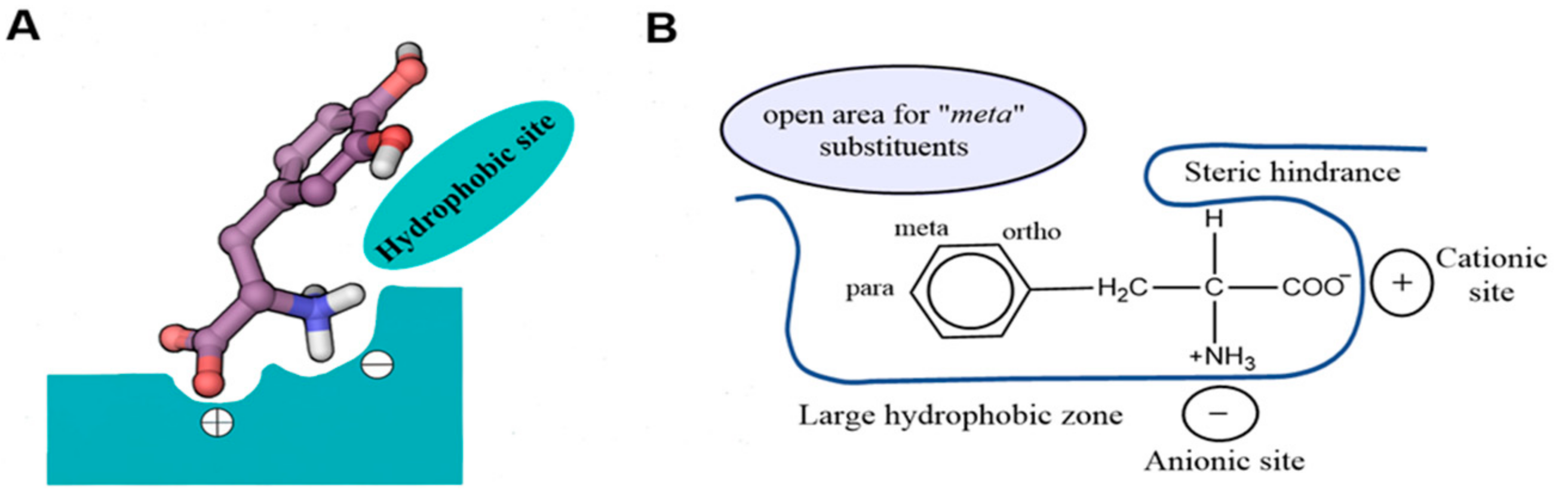
As another important difference between hydrophobic and hydrophilic amino acids, hydrophobic ones occur at the centre of proteins while hydrophilic amino acids are on the surface. Moreover, hydrophobic amino acids have long side chains with mostly carbon and hydrogen atoms whereas hydrophilic amino acids have either short side chains or side chain with hydrophilic groups. Hence, this is the key difference between hydrophobic and hydrophilic amino acids. Hydrophobic amino acids are a type of amino acids which have a nonpolar nature while hydrophilic amino acids are a type of amino acids in which have a polar nature. What is the Difference Between Hydrophobic and Hydrophilic Amino Acids? Since water is a polar solvent and these amino acids are also polar, they can dissolve in water. The name “hydrophilic” derives because it attracts water.
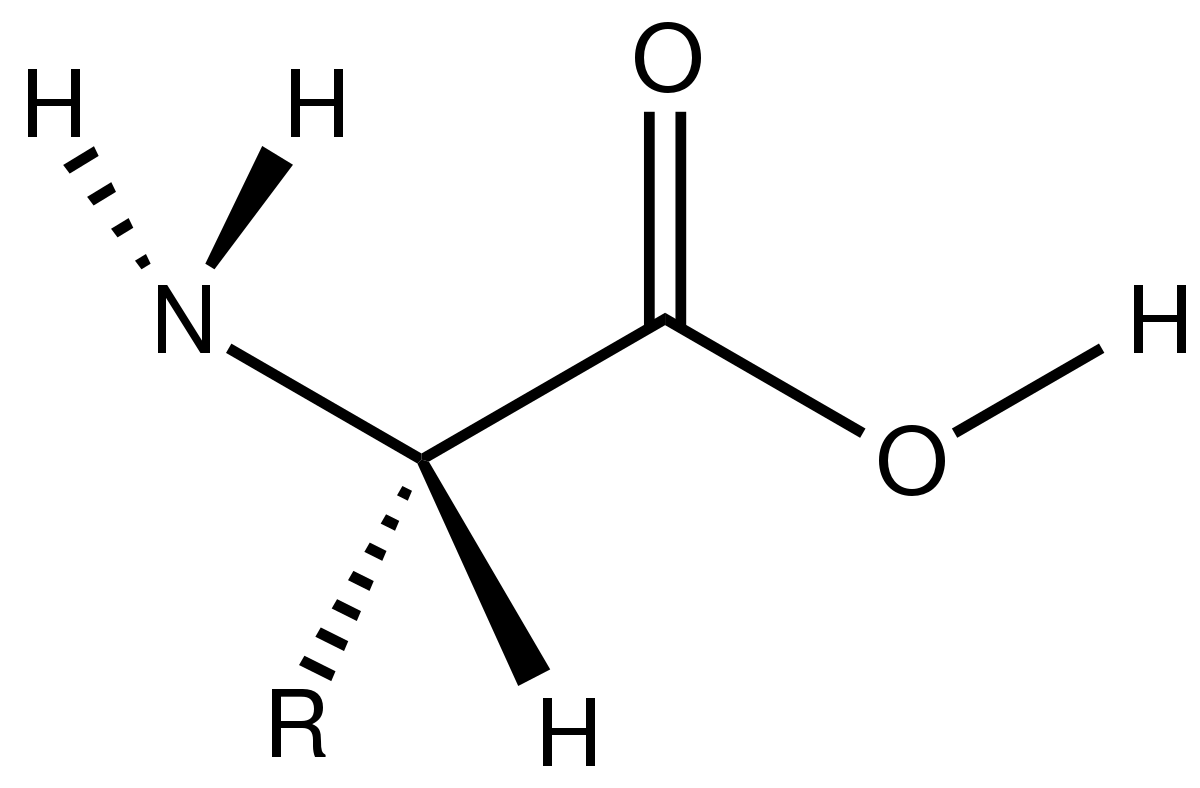
Hydrophilic amino acids are a type of amino acids with a polar nature. Moreover, the hydrophobic amino acids among essential amino acids are as follows. Therefore, they tend to repel from the water. Furthermore, they have small dipole moments. Thus, if the side chain is very long and consists mostly of carbon and hydrogen atoms, they are hydrophobic. This R group can be simply an atom (hydrogen atom) or a long side chain. An amino acid has the general formula in which a central carbon atom is attached with a hydrogen atom, a carboxyl group, an amine group and a side group (R group). Hence, the hydrophobic nature of these compounds arises due to the side chains they have in their chemical structure. Since these amino acids are nonpolar, they cannot dissolve in water. Likewise, the name “hydrophobic” derives because it does not interact with water (“hydro” – water). Hydrophobic amino acids are a type of amino acids with a nonpolar nature. Summary What are Hydrophobic Amino Acids? Side by Side Comparison – Hydrophobic vs Hydrophilic Amino Acids in Tabular Formĥ. They are different from each other mainly based on the polarity. Moreover, we can categorize them as hydrophilic and hydrophobic amino acids, depending on their physicochemical nature. Furthermore, amino acids are mainly in two types as essential and nonessential amino acids. A protein is a giant polymer molecule which is an essential component of all living organisms. The key difference between hydrophobic and hydrophilic amino acids is that the hydrophobic amino acids are nonpolar whereas the hydrophilic amino acids are polar.Īmino acids are the building blocks of proteins.


 0 kommentar(er)
0 kommentar(er)
